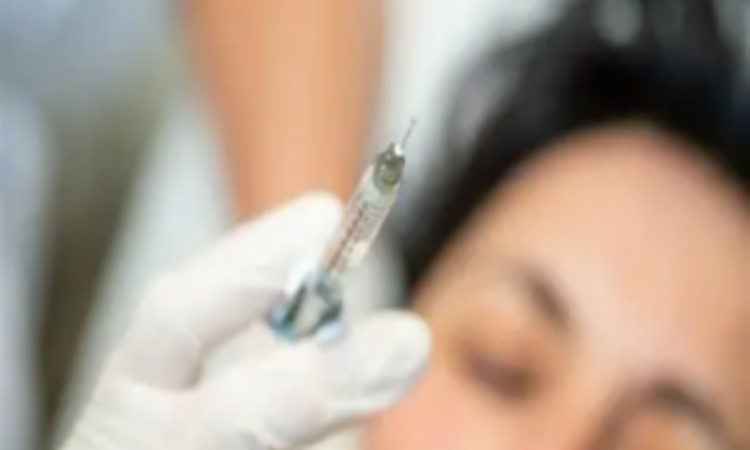
People hospitalised following suspected use of unlicensed botulinum toxin products.
The Medicines and Healthcare products Regulatory Agency (MHRA) is warning criminals that they face prison as it cracks down on the illicit trade in unlicensed botulinum toxin products, commonly referred to as Botox, used in cosmetic procedures.
The MHRA’s Criminal Enforcement Unit has launched a number of criminal investigations following a spike in hospital admissions believed to be linked to the use of unlicensed botulinum toxin products.
Between 4 June and 6 August 2025, 41 confirmed cases of botulism – a rare but potentially life-threatening condition causing paralysis – were reported across several regions in England, including the North East, East Midlands, East of England, North West, and Yorkshire and Humber.
The Criminal Enforcement Unit has seen evidence that some sellers and practitioners – often untrained – are obtaining unlicensed botulinum toxin products illegally and offering injections in unsafe, unregulated settings. The treatments are being delivered in informal settings such as domestic bedrooms and kitchens, hair salons, and through mobile beauty services. Members of the public are often lured in by adverts on social media promising quick, cheap results.
The Criminal Enforcement Unit is investigating the illegal trade in these products. Anyone caught selling or supplying unlicensed botulinum toxin faces up to two years in prison and unlimited fines under the Human Medicines Regulations 2012.
Andy Morling, Head of the MHRA’s Criminal Enforcement Unit, said:
“Criminals are exploiting the popularity of cosmetic treatments by peddling dangerous, unlicensed products, putting profit before safety.
“Anyone involved in the supply of unlicensed botulinum toxin – whether through organised networks or informal sales from kitchen tables, hair salons, or via social media – is breaking the law and endangering lives. The 41 individuals we’ve seen between June and August left seriously ill represent the devastating human cost of this trade.
“We are working across the country to identify those responsible, seize illegal products, and bring cases to court. We use the full range of our enforcement powers and techniques to shut down these operations and bring offenders to justice.”
This crackdown is part of the MHRA’s wider work to disrupt illegal botulinum toxin supply. Since May 2023, the Criminal Enforcement Unit, working closely with its partners in Border Force, has seized more than 4,700 vials of unlicensed botulinum toxin both at the border and inland.
Almost all of the seized products originated in South Korea, including brands such as Botulax, reNTox, Innotox, and Toxpia, which are not authorised for sale in the UK.
The Criminal Enforcement Unit also works with social media companies to remove illegal listings and disrupt criminal networks advertising unlicensed botulinum toxin.
Understanding the health risks
Botulism caused by botulinum toxin in cosmetic procedures is rare, but can be life-threatening. Symptoms can take up to four weeks to develop and may include difficulty swallowing, slurred speech and breathing difficulty. In severe cases, patients may require mechanical ventilation and intensive care treatment.
Anyone who has recently received a botulinum toxin treatment and develops any of these symptoms should seek medical help immediately via NHS 111 or emergency services.
Health Minister Stephen Kinnock said:
“No one should have to suffer serious illness or risk their life because criminals are flooding the market with unsafe products.
“This government is determined to crack down on cosmetic cowboys who exploit vulnerable consumers seeking cut-price treatments outside suitable medical settings. Through the MHRA’s criminal investigations and our new regulations, we’ll use the full force of the law against those who supply unlicensed medicines.
“I would urge anyone considering a cosmetic procedure to consider the risks and find a reputable, insured, and qualified practitioner.”
MHRA Chief Safety Officer Dr Alison Cave said:
“Public safety is a top priority for the MHRA. Botulinum toxin is a prescription-only medicine and should only be sold or supplied in accordance with a prescription given by an appropriate prescriber such as a doctor or other qualified healthcare professional.
“Buying botulinum toxin in any other circumstances significantly increases the risk of getting a product which is either falsified or not licensed for use in the UK. This means that there are no safeguards to ensure products meet the MHRA’s standards for quality and safety. As such, they can have life-threatening consequences for the people who take them.
“If you are offered botulinum toxin without a medical consultation, in an informal setting, or at a price significantly below usual rates, this should be treated as a warning sign. Lower cost does not mean safe; it may put your health at risk and could lead to hospitalisation.”
How to protect yourself and report concerns
Licensed botulinum toxin products undergo rigorous testing and quality controls to ensure they contain the correct active ingredient at safe concentrations. Legitimate treatments should only be carried out in proper clinical settings with appropriate emergency equipment available, and must be prescribed for individual patients either directly or under a Patient Specific Direction (PSD).
Before any treatment, verify that your administering practitioner is appropriately trained and qualified. Check that products being used are licensed in the UK by asking to see packaging and checking batch numbers. Be suspicious of unusually cheap prices, treatments offered in domestic settings, or practitioners who cannot provide proper credentials.
The MHRA urges anyone who experiences side effects or complications after a cosmetic procedure to report them via the Yellow Card scheme at https://yellowcard.mhra.gov.uk/.
Notes
- This follows safety reports published by the UK Health Security Agency of 41 confirmed cases of botulism between 4 June and 6 August 2025 in regions across England.
- The Medicines and Healthcare products Regulatory Agency (MHRA) is responsible for regulating all medicines and medical devices in the UK by ensuring they work and are acceptably safe. All our work is underpinned by robust and fact-based judgements to ensure that the benefits justify any risks.
- The MHRA is an executive agency of the Department of Health and Social Care.
source: GOV

 ENFIELD
ENFIELD HACKNEY
HACKNEY HARINGEY
HARINGEY ISLINGTON
ISLINGTON