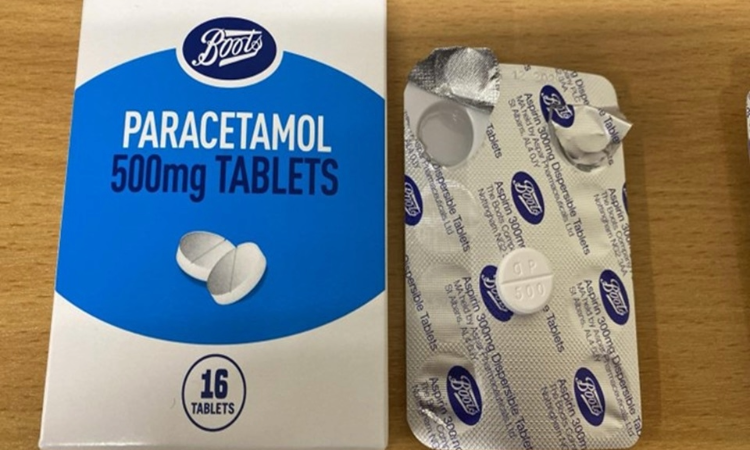
People who have purchased Boots Paracetamol 500mg Tablets 16s (Item code 81-99-922, Batch 241005, Expiry date 12/2029) are advised to stop using the product immediately and return it to a Boots store for a full refund, because of a packaging error.
The Medicines and Healthcare products Regulatory Agency (MHRA) has issued a medicines recall alert due to a packaging error where the foil blister inside the carton incorrectly states ‘Aspirin 300mg Dispersible Tablets’ instead of ‘Paracetamol 500mg Tablets’. The Boots Company PLC and the supplier, Aspar Pharmaceuticals Limited, have confirmed that the tablets in the blister packs are Paracetamol 500mg and not aspirin, and are conducting a full investigation into the issue.
- To read the article in Turkish: https://olaygazete.co.uk/ingiltere-gundemi/boots-paracetamol-500-mg-tablet-alanlar-ambalaj-hatasi-nedeniyle-iade-cagrisi.html
Members of the public, including carers, should check if their pack has the batch number 241005, which can be found on the bottom of the box. If affected, they should stop using the product immediately and return it to a Boots store for a full refund, with or without receipt.
Boots Paracetamol 500mg packs, with the batch number 241005, should not be kept at home, even if the error is known, as this could lead to confusion and an incorrect dose being taken. Anyone who has purchased this product for someone else should inform them as soon as possible.
Dr Stephanie Millican, MHRA Deputy Director Benefit Risk Evaluation, said:
Patient safety is always our priority. It is vitally important that you check the packaging of your Boots Paracetamol 500mg Tablets 16s, and if the batch number is 241005, you should stop using the product and return it to a Boots store for a full refund.
If you are unsure which pack you have purchased or have taken Boots Paracetamol 500mg Tablets and experienced any side effects, seek advice from a healthcare professional. Please report any suspected adverse reactions via the MHRA’s Yellow Card scheme.
If you have any questions or require further advice, please seek advice from your pharmacist or other relevant healthcare professional.
Advice for Members of the Public:
- Stop using the impacted batch immediately and return this to Boots stores where a full refund will be provided with or without a receipt.
- Aspar Pharmaceuticals Limited and The Boots Company PLC have confirmed that the tablets in the blister packs are Paracetamol 500mg and not aspirin. If you have taken tablets from this batch and have any additional questions, please seek advice from your pharmacist or other relevant healthcare professional.
- Patients who experience any suspected adverse reactions or have any questions about the medication should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card Scheme.
Notes:
- The MHRA has issued a recall notification for a specific batch of Boots Paracetamol 500mg Tablets due to a packaging error: Class 2 Medicines Recall Notification: Boots Paracetamol 500 mg tablets (16s)
- This recall affects 119,964 packs of Boots Paracetamol 500mg (16s)
- The Medicines and Healthcare products Regulatory Agency (MHRA) is responsible for regulating all medicines and medical devices in the UK by ensuring they work and are acceptably safe. All our work is underpinned by robust and fact-based judgements to ensure that the benefits justify any risks.
- The MHRA is an executive agency of the Department of Health and Social Care.
- The Yellow Card Scheme is MHRA’s system of monitoring the safety of medicines in the UK and it acts as an early warning system to identify new, and strengthen existing, safety information about medicines. Yellow Cards are used alongside other scientific safety information to help MHRA to take action, if necessary, to make changes to the warnings given to people taking a medicine or review the way the medicine is used to maximise benefit and minimise the risk to the patient.
source: GOV



 ENFIELD
ENFIELD HACKNEY
HACKNEY HARINGEY
HARINGEY ISLINGTON
ISLINGTON